There was an exchange meeting about how to analyze the data of Alibaba website in our company on Apr.7th, 2016. The participants whose company is the one of best in their field were very positive to share their experience.
publisherxiong
time2016/08/06

- There was an exchange meeting about how to analyze the data of Alibaba website in our company on Apr.7th, 2016. The participants whose company is the one of best in their field were very positive to share their experience.
Despite the lack of a breakthrough in battery technology, the capacity and energy density of lithium-ion technology has crept up slowly but surely. Now you could quite easily be carrying around three or four thousand milliamp hours in your pocket. That’s a lot of energy to be released if something goes wrong, and did I mention it’s in your pocket? Scientists from Stanford University have developed a doping process that could make future batteries safer and less prone to wearing out.
Modern lithium-ion batteries are composed of multiple cells, and within each of them are cathodes and anodes. If a battery is physically damaged or over-charged, the barrier between these halves can be weakened to the point of failure, resulting in a short that can cause an exothermic reaction. This can spread and rupture other cells in a process known as thermal runaway. The end result is a fire or explosion that releases all that energy. Hopefully you’re not holding it when that happens.
One reason this damage can occur in batteries is from the formation of dendrites, finger-like deposits of lithium metal that can pierce the barrier between electrodes. This build up gets worse as the battery is depleted and recharged, and it also contributes to loss of capacity. According to the Stanford research, it may be possible to add a few more compounds to the electrolyte solution and do away with dendrite formation.
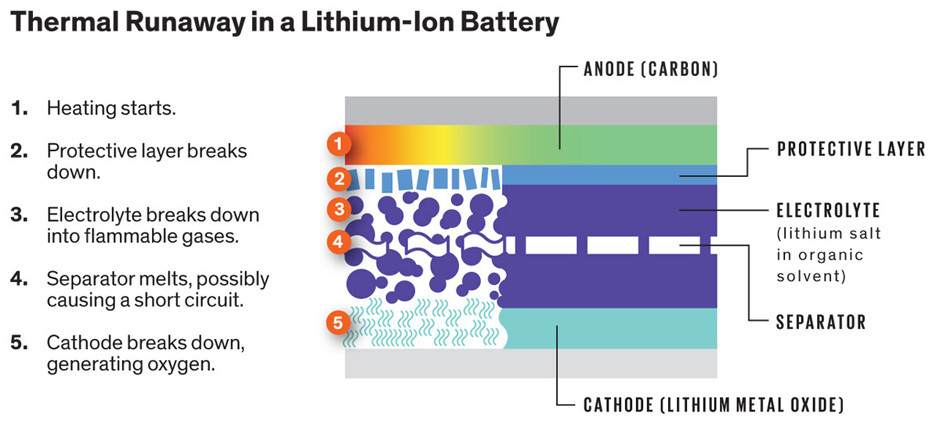
One additive, lithium nitrate has been researched in the past as a way to boost battery performance. The second additive used in the study is lithium polysulfide, which is seen as a contaminant in some areas of research. If forms from the breakdown of sulfur electrodes and can cause damage to lithium metal electrodes. However, when paired with lithium nitrate, they form a dendrite-busting solution that coats and protects electrodes. Instead of dendrites, you get flat deposits of lithium that don’t cause damage to the battery.
The research was conducted on small coin cell batteries, not the giant cells found in your phone or tablet. Still, the principal is the same. And as a nice bonus, the treated batteries maintained their capacity better. After 300 discharge cycles, the doped batteries still had 99% efficiency, whereas a battery doped with only lithium nitrate was down to 92% after just 180 cycles.
This technique could be valuable in the ongoing development of lithium-air and lithium-sulfur rechargeable batteries, which are susceptible to dendrite formation. They could also have ten times the capacity of current batteries. You certainly don’t want that exploding in your pocket. You know what they say — with great power comes great responsibility.
